Follow along with me: Parkinson’s Disease 101 will be a four-part series!
For the next two months I’ll be writing about Parkinson’s in an effort to raise awareness and money for this devastating neurodegenerative disease. I’ll be walking in this year’s Parkinson’s Foundation Moving Day fundraiser. Please support me by clicking the button below to donate!
The Series So Far
In case you are just joining us, here are the links to the previous posts. They are meant to be read in order, starting with the first one:
The Goal
Last year I published a scientific paper in the journal Brain that investigated Parkinson’s disease. What started out as a “bite sized” project for my postdoctoral fellowship turned into a collaborative and amazing learning experience. I learned so much during this process, and I want to impart some of this knowledge onto you! Scientific papers can be so full of jargon and technical terminology, making them a little intimidating to read. Therefore, my goal is to break down this paper into digestible short posts to take away the most important information.
If you are feeling brave, you can read the original article: link here. Otherwise, let’s start with discussing the role of genetics and Parkinson’s disease.
The Gene in Question: LRRK2 G2019S
If you were to ask scientists 25 years ago what causes Parkinson’s disease, many of them would point to the environment, mainly pollution and pesticides, as the main culprit. Today, we see a wealth of evidence that points to genetics as another driving force.
In the early 2000s, researchers discovered a gene variant, also called a mutation, in the LRRK2 gene on chromosome 12.1 In the LRRK2 gene, a simple substitution of one of the letters in our DNA—from a G to an A—was found to increase one’s risk of developing Parkinson’s disease. This one letter substitution in our DNA is called a single nucleotide polymorphism, or SNP for short. Since then, there’s been a mad dash to understand why this genetic variant in LRRK2 results in an increased risk of PD, as well as other SNPs that may also increase our risk. The LRRK2 gene codes for the LRRK2 protein, and understanding the altered function of LRRK2 resulting from the mutation could unlock mysteries as to why neurons from the substantia nigra degenerate in Parkinson’s and get us closer to developing disease-modifying therapies.
The LRRK2 protein is a special type of protein called a kinase. This is a fancy way of saying that LRRK2 helps activate other proteins. Let’s use an analogy to explain. Recall your days of playing duck-duck-goose in the school yard. Everyone is sitting in a circle except one, who is diligently walking around the circle tapping each person on the head and saying “duck”. Eventually, someone will get tapped on the head, “goose!” will be shouted, and there will be a frenzy of activity as the person tagged tries to catch the perpetrator. LRRK2 is like that person circling the group, but it is instead circling the cell and activating other proteins that it touches (“goose”!).
Now, there was always that wild kid on the playground who would run around the circle, defying all the normal rules and decorum, and tapping everyone on the head and yelling “goose!”. A mad frenzy would ensue. Everyone jumping up; kids running into each other; pure and utter chaos!
This lack of control what is happening when that that G gets substituted for an A, causing a LRRK2 G2019S variant. There is an above average activation of proteins in neurons that causes issues. Scientists are still trying to figure out exactly how this over activation of proteins in the cell may predispose an individual to Parkinson’s disease, but so far the evidence suggests a complex story impacting several different processes that occur in neurons. These include disruptions to how neurons generate energy, transport molecules/proteins across the cell, and clean up waste products; all these disruptions result in increased stress on neurons and increases the chance that they will degenerate.
The Study
Ok, we now have enough background to start discussing the study! In 2018, the 23andMe research team launched the Parkinson’s Impact Project (PIP) with the goal of better understanding the symptoms of LRRK2 G2019S carriers with Parkinson’s disease (PD) and comparing these symptoms to participants who do not carry the LRRK2 G2019S variant but still developed PD. So far, evidence from scientific publications suggested that the symptoms and the progression of PD in LRRK2 G2019S carriers were the same as those without the variant, often referred to as idiopathic PD. By examining the symptom profiles of these participants at their baseline survey, we were able to challenge this notion.
More specifically, LRRK2 G2019S carriers with PD reported less cognitive and memory issues, less loss of smell, and fewer cases of REM sleep behavior disorder (RBD) compared to participants with PD but who do not carry LRRK2 G2019S. The figure below depicts these findings in more detail:
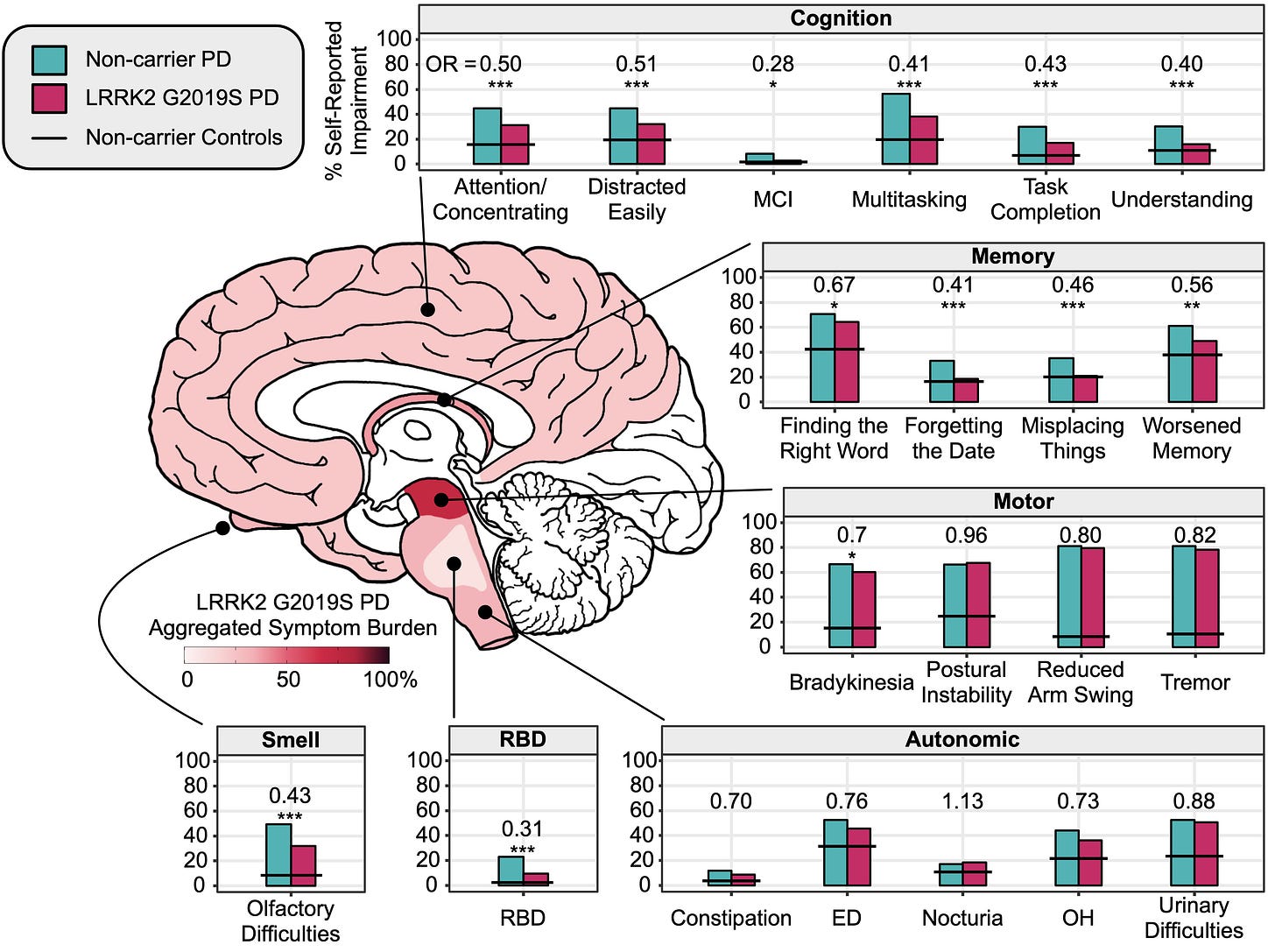
What we can see from this figure is that the percentage of self-reported impairment for motor symptoms are quite similar (i.e., the bars are the same height). However, when we inspect the height of the bars for cognition, memory, smell, and RBD, we see that LRRK2 G2019S carriers reported less impairment for these symptoms compared to non-carriers with PD (i.e., idiopathic PD). These differences already include adjustments for participants’ age, sex, education, and the number of years diagnosed with PD.
Why is this important? We learned in a previous post about prodromal symptoms and how certain symptoms, like loss of smell and RBD, can signal an increased risk for PD. LRRK2 G2019S carriers may not exhibit these symptoms; however, they may still go on to develop the motor symptoms classically associated with PD. In other words, how symptoms develop in LRRK2 G2019S carriers may be different than non-carriers.
Lastly, clinical trials may need more sensitive criteria used to determine the progression of PD when recruiting LRRK2 G2019S carriers. Relying on prodromal markers to identify early onset cases may not apply directly for LRRK2 G2019S carriers.
Summary
The LRRK2 G2019S genetic variant increases the risk of developing Parkinson’s disease. Our recent study observed that LRRK2 G2019S carriers report less prodromal symptoms of Parkinson’s, like loss of smell and REM sleep behavior disorder, when compared to non-carriers with PD. These differences in symptom profile may be important for establishing risk and designing clinical trials.
For the next two months I’ll be writing about Parkinson’s in an effort to raise awareness and money for this devastating neurodegenerative disease.
I’ll be walking in this year’s Parkinson’s Foundation Moving Day fundraiser. Please support me by clicking the button below to donate!
Following convention, I will italicize genes and regular case the proteins.




Thank you for these. My FIL was diagnosed Monday and these articles are very helpful